Newswise — Every plant, animal, and person is a rich microcosm of tiny, specialized cells. These cells are worlds unto themselves, each with their own unique parts and processes that elude the naked eye. Being able to see the inner workings of these microscopic building blocks at nanometer resolution without harming their delicate organelles has been a challenge, but scientists from different disciplines across the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have found an effective way to image a single cell using multiple techniques. The fascinating process to capture these images was published in Communications Biology.
Being able to understand the inner structures of cells, the way chemicals and proteins interact within them, and how those interactions signal certain biological processes at nanometer resolution can have significant implications in medicine, agriculture, and many other important fields. This work is also paving the way for better biological imaging techniques and new instruments to optimize biological imaging.
“Studying human cells and the organelles inside of them is exciting,” said Qun Liu, a structural biologist at Brookhaven Lab, “but there are so many opportunities to benefit from our multimodal approach that combines hard X-ray computed tomography and X-ray fluorescence imaging. We can study pathogenic fungi or beneficial bacteria. We’re able to not only see the structure of these microorganisms but also the chemical processes that happen when cells interact in different ways.”
Pulling out one of life’s building blocks
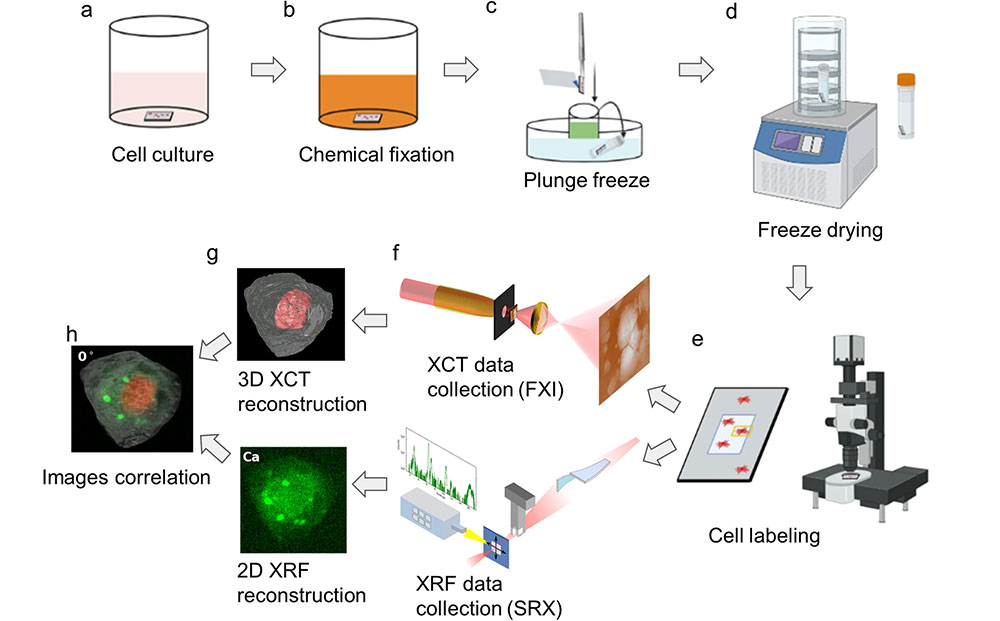
Before the researchers even began imaging, one of their biggest challenges was preparing the sample itself. The team decided to use a cell from the human embryonic kidney (HEK) 293 line. These cells are known for being easy to grow but difficult to take multiple X-ray measurements of. Even though they are very small, cells are quite susceptible to X-ray-induced damage.
The scientists went through a careful, multistep process to make the sample more robust. They used paraformaldehyde to chemically preserve the structure of the cell, then had a robot rapidly freeze the samples by plunging them into liquid ethane, transferred them to liquid nitrogen, and finally freeze dried them to remove water but maintain the cellular structure. Once this process was complete, the researchers placed the freeze-dried cells under a microscope to locate and label them for targeted imaging.
At only about 12-15 microns in diameter (the average human hair is 150 microns thick), setting up the sample for measurements was not easy, especially for measurements on different beamlines. The team needed to ensure that the cell’s structure could survive multiple measurements with high energy X-rays without significant damage and that the cell could be reliably held in one place for multiple measurements. To overcome these hurdles, the scientists created standardized sample holders to be used on multiple pieces of equipment and implemented optical microscopes to quickly find and image the cell and minimize prolonged X-ray exposure that could damage it.
Multimodal measurements
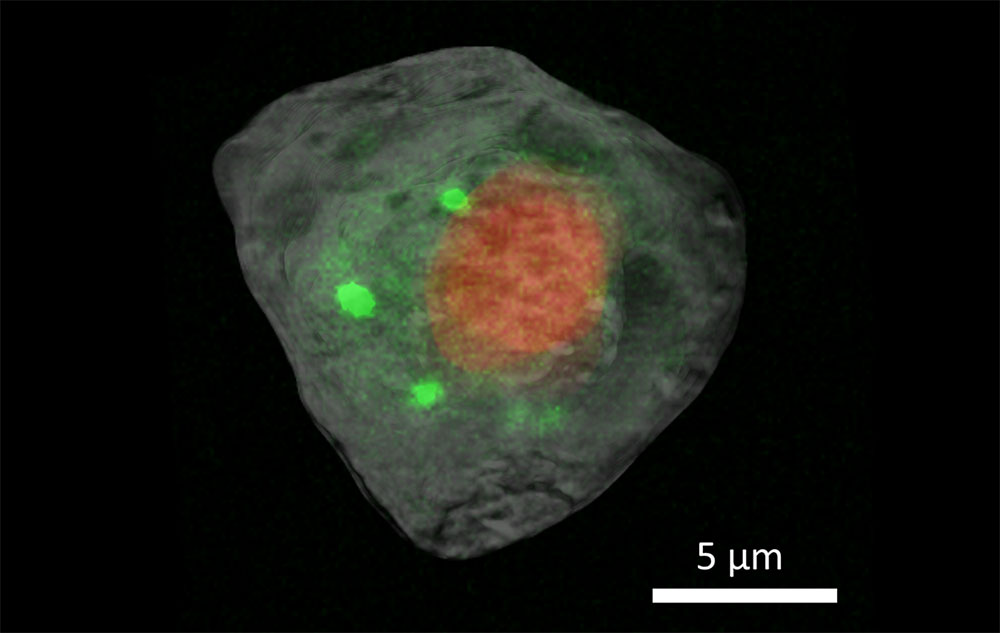
The team used two imaging techniques found at the National Synchrotron Light Source II (NSLS-II) — a DOE Office of Science user facility at Brookhaven — X-ray computed tomography (XCT) and X-ray fluorescence (XRF) microscopy.
The researchers collected XCT data, which uses X-rays to tell scientists about the cell’s physical structure, on the Full Field X-ray Imaging (FXI) beamline. Tomography uses X-rays to show a cross-section of a solid sample. A familiar example of this is the CT scan, which medical practitioners use to image cross sections of any part of the body.
The researchers collected XRF microscopy data, which provides more clues about the distribution of chemical elements within the cell, on the Submicron Resolution X-ray Spectroscopy (SRX) beamline. In this technique, the researchers direct high energy X-rays at a sample, exciting the material and causing it to emit X-ray fluorescence. The X-ray emission has its own unique signature, letting scientists know exactly what elements the sample is composed of and how they are distributed to fulfill their biological functions.
“We were motivated to combine XCT and XRF imaging based on the unique, complementary information each provides,” said Xianghui Xiao, FXI lead beamline scientist. “Fluorescence gives us a lot of useful information about the trace elements inside of cells and how they are distributed. This is very critical information to biologists. Getting a high-resolution fluorescence map on many cells can be very time consuming, though. Even just for a 2D image, it may take quite a few hours.”
This is where getting a 3D image of the cell using XCT is helpful. This information can help guide the fluorescence measurements to specific locations of interest. It saves time for the scientists, increasing throughput, and it also ensures that the sample doesn’t need to be exposed to the X-rays for as long, mitigating potential damage to the fragile cell.
“This correlative approach provides useful, complementary information that could advance several practical applications,” remarked Yang Yang, a beamline scientist at SRX. “For something like drug delivery, specific subsets of organelles can be identified, and then specific elements can be traced as they are redistributed during treatment, giving us a clearer picture of how these pharmaceuticals work on a cellular level.”
While these advances in imaging have provided a better view into the cellular world, there are still challenges to be met and ways to improve imaging even further. As part of the NSLS-II Experimental Tools III project — a plan to build out new beamlines to provide the user community with new capabilities — Yang is science lead of the team working on the upcoming Quantitative Cellular Tomography (QCT) beamline, which will be dedicated to bio-imaging. QCT is a full-field soft X-ray tomography beamline for imaging frozen cells with nanoscale resolution without the need for chemical fixation. This cryo-soft X-ray tomography beamline will be complementary to current methods, providing even more detail into cellular structure and functions.
Future findings
While being able to peer into the cells that make up the systems in human bodies is fascinating, being able to understand the pathogens that attack and disrupt those systems can give scientists an edge in fighting infectious disease.
“This technology allows us to study the interaction between a pathogen and its host,” explained Liu. “We can look at the pathogen and a healthy cell before infection and then image them both during and after the infection. We will notice structural changes in both the pathogen and the host and gain a better understanding of the process. We can also study the interaction between beneficial bacteria in the human microbiome or fungi that have a symbiotic relationship with plants.”
Liu is currently working with scientists from other national laboratories and universities for DOE’s Biological and Environmental Research Program to study the molecular interactions between sorghum and Colletotrichum sublineola, the pathogenic fungus that causes anthracnose, which can harm the leaves of plants. Sorghum is a major DOE bioenergy crop and is the fifth most important cereal crop in the world, so humanity would have a lot to gain by understanding the tactics of this devastating fungus and how sorghum’s defenses operate at the cellular and molecular levels.
Being able to see at this scale can give scientists insight into the wars being waged by pathogens on crops, the environment, and even human bodies. This information can help develop the right tools to fight these invaders or fix systems that aren’t working optimally at a fundamental level. The first step is being able to see a world that human eyes aren’t able to see, and advances in synchrotron science have proven to be a powerful tool in uncovering it.
This work was supported by Brookhaven’s Laboratory Directed Research and Development funding and the DOE Office of Science.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.