Newswise — Fuel cells are quickly becoming a viable, clean energy alternative to commonly used fossil fuels, such as gasoline, coal, and oil. Fossil fuels are non-renewable energy resources that release carbon dioxide into the atmosphere. Fuel cells, however, rely on an electrochemical reaction rather than combustion, producing carbon-free energy. One of the barriers to scaling this technology up to be commercially viable is the current reliance on platinum group metals (PGM) as catalysts. Due to their high cost and limited supply, PGMs often account for 46% of the production cost of fuel cells.
To help address this particular challenge, researchers at Purdue University, the U.S. Department of Energy’s (DOE) Oak Ridge National Laboratory, and DOE’s Brookhaven National Laboratory investigated iron-nitrogen doped carbon (Fe-N-C) catalysts as an effective alternative to PGM-based catalysts. In this study, the researchers used a newly developed high-energy resolution X-ray spectroscopy technique at the Inner-Shell Spectroscopy (ISS) beamline at the National Synchrotron Light Source II (NSLS-II), a DOE Office of Science user facility at Brookhaven. The researchers were able to analyze the electronic structure of this catalyst material with the addition of the ionomer Nafion, a material needed to control the movement of charged particles (ions). The results, recently published in ACS Applied Energy Materials, have given researchers new insight into the behavior of these materials, helping to refine their search for a low-cost PGM alternative with high activity, selectivity, and stability.
“Fe-N-C systems have been intensely studied by multiple research groups,” said Yulia Pushkar, a professor of physics at Purdue University and the lead author of this paper. “However, the underpinning of the true catalytic center, which would contain an iron atom but perform as good as platinum in an oxygen reduction reaction, has never been completely established in this highly promising class of materials. The challenge and mystery of this problem attracted my attention.”
A greener, cleaner fuel alternative
To understand why these catalysts are so important, it helps to know a little more about how fuel cells work. A fuel source, like hydrogen, will enter the system on the negative electrode (“anode”) side. The catalyst at the anode then splits the hydrogen molecule into positively charged protons and negatively charged electrons. The electrons are released through an external circuit while the protons pass through an electrolyte material that does not let electrons through. At the cathode, the positive end of the cell, the catalyst combines the protons and electrons with oxygen in the air. The reaction, known as an oxygen reduction reaction, releases energy and, as a byproduct, water.
Hydrogen also has a high energy density—three times higher than that of gasoline. Being able to efficiently harness the power of hydrogen could be a significant step in the pathway to reduce carbon emissions. Finding the right material to scale up catalyst production has posed a significant challenge, though.
There are several hydrogen powered fuel cell technologies currently being developed, but proton-exchange membrane fuel cells appear to be the most promising. They are easy to make, operate at relatively low temperatures, and perform efficiently. The most effective catalyst materials for these fuel cells, however, are made of PGMs, which are excellent electrocatalysts, but their limited supply and high cost prohibit large scale production. Researchers have been hard at work looking for low-cost alternatives that not only provide comparable performance but are also as stable and robust. This is particularly relevant in applications like electric vehicles, where the performance demand is quite high.
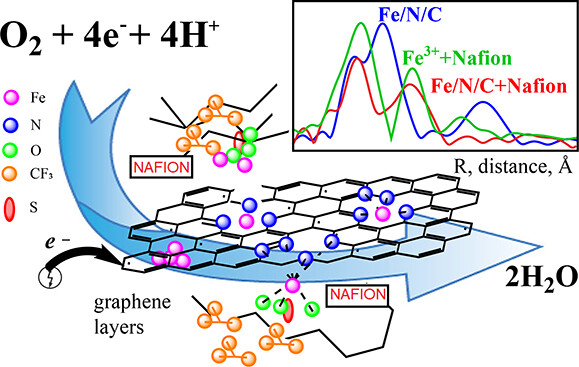
To address this issue, the team decided to take a closer look at Fe-N-C, a promising candidate in a class of catalyst materials called metal-nitrogen doped carbon. Fe-N-C is produced by inserting iron atoms into graphene sheets, single layers of carbon atoms arranged in a hexagonal lattice pattern. To further improve performance, some of the carbon atoms in the graphene are then replaced with nitrogen atoms. The performance of the Fe-N-C catalyst was comparable to the PGM catalysts that are currently in use, but its durability did not fare as well. The team needed to understand the mechanism behind this catalyst’s degradation in order to improve its stability.
To improve stability, the team also looked at what would happen if they added a polymer called Nafion to the Fe-N-C catalyst. Nafion is a commonly used ionomer, a stable, highly conductive polymer that is resistant to the acidic environment and found in most fuel cells.
Peering in with a higher resolution
To get an accurate picture of the reactions happening within the Fe-N-C catalyst, the team used several powerful synchrotron-based x-ray spectroscopy techniques. The researchers performed X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) studies at beamline 20-BM at the Advanced Photon Source, a DOE Office of Science user facility at DOE’s Argonne National Laboratory. The team performed X-ray Emission Spectroscopy (XES) at the ISS beamline at NSLS-II. XES is a technique that gives researchers valuable information regarding a material’s electronic structure.
“With XES, tiny changes in a material’s chemical state associated with catalytic activity can be revealed,” explained Eli Stavitski, lead beamline scientist at ISS. “Traditional X-ray spectroscopy is not sensitive to the spin state, which is a magnetic moment created by the electron arrangement in the molecule.. XES, however, provides this kind of insight. We determined that the active complex is present in high spin configuration, meaning it has more electron momentum. In these experiments, we also probed the oxidation state and surrounding ligands of the iron atom in the Fe-N-C catalyst. We were able to see the oxidation state changes when driving the catalytic reaction and its precise determination. This is critical to understanding reaction mechanisms.”
This was the one of the first experiments using the beamline’s new high-resolution x-ray spectrometer. It was designed and built at NSLS-II, with ISS beamline scientist Denis Leshchev leading the project. At the heart of the spectrometer are crystal analyzers — ultra-pure, thin silicon wafers that are precisely cut, polished to perfection, and bent into a shape that allows them to condense photons into small, tight spots like a powerful x-ray lens. Pushkar’s team has developed a unique assembly of large silicon crystal analyzers which, when coupled with the beamline’s intense x-ray beam, precision mechanics, and the detector, made this experiment possible.
“When the X-ray beam from NSLS-II interacts with the sample, the sample emits characteristic x-rays, which are traditionally used to fingerprint the elemental composition of the sample,” explained Leshchev. “X-ray spectroscopy analyzes the interactions between the X-ray beam and the sample, and the technique probes not only the presence of elements, but also their atomic environment. The new, high-resolution spectrometer further enhances the ability of an experiment to resolve fine details of these interactions and offers detailed insights into connections between the atomic properties of materials and their catalytic performance.
“This setup allows for a more precise characterization of energy related materials, like catalysts and other battery materials,” said Leshchev. “Traditional X-ray absorption spectroscopy is a common technique at many synchrotrons. It is now extending into high-resolution spectroscopy. We’re excited to be able to offer this capability to our users now.” The team used these techniques to study the behavior of the Fe-N-C catalyst during an oxidation reduction reaction with and without the presence of Nafion. They found that adding Nafion caused significant changes, particularly in terms of the oxidation state of the iron atoms and their interactions with neighboring atoms.
They found that catalytically active iron atoms in the Fe-N-C catalysts tend to be in a specific state — ferric ion (Fe3+)high spin centers surrounded by nitrogen atoms. When these catalysts are mixed with Nafion, the ionomer releases some of the iron atoms that are bound too strongly to the graphite sheet, allowing them to participate in the catalytic process. Nafion is an essential component in experimental and industrial fuel cells because it brings protons to the catalytic site for water formation. Understanding the Nafion-catalyst interaction is essential to optimizing fuel cell performance.
“We are still in the process of answering the central question that led us to this research,” said Pushkar, “but we’ve uncovered an additional layer of complexity in this system. The strong interaction of Nafion — currently an indispensable component — with iron centers in the system causes a re-structuring of the iron ligand environments.”
This observation is important for designing better catalysts because it addresses the questions about which forms of iron are actually the most effective in catalyzing the oxidation reduction reaction process. Experiments like this help bring fuel cell researchers closer to an ideal catalyst with high performance and stability, while improving cost and availability to allow this clean energy alternative to make a significant impact on scaling back carbon emissions.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.