Chula researchers have developed a remarkable wireless hepatitis B virus test kit to screen for infection and collect data for an online database that’s fast and complete in one step. Industrial production is targeted for use throughout the country.
Newswise — Hepatitis B is a disease that causes illness and death in a large population and causes chronic liver diseases such as cirrhosis and liver cancer, the most common cancer in Thailand and in the world. For Thailand, the latest data from the Ministry of Health shows that the country currently has more than 2 million people infected with chronic hepatitis B!
Therefore, hepatitis B is considered a major public health disease. The World Health Organization (WHO) even announced a campaign that aims to eradicate hepatitis B by 2030 or just 6 years from now.
With the situation of hepatitis B in Thailand combined with the WHO campaign, a group of researchers from Chulalongkorn University led by Prof. Pisit Tangkijvanich, M.D. and Dr. Natthaya Chuaypen from the Faculty of Medicine, together with Professor Dr. Orawon Chailapakul, and Dr. Prinjaporn Teengam from the Department of Chemistry, the Faculty of Science, have jointly developed a Wireless Point-of-Care Testing for Hepatitis B Virus infection to help make the diagnosis of hepatitis B simpler and faster.

Why is early diagnosis of hepatitis B important?
Prof. Pisit Tangkijvanich, M.D., Head of the Center of Excellence in Hepatitis and Liver Cancer, Faculty of Medicine, Chulalongkorn University, and former President of the Thai Association for the Study of the Liver (THASL) 2021-2022 discusses the situation of hepatitis B ten years ago, of which statistics is still very close to today.
“About 2-4 percent of the total population of Thailand has hepatitis B. The majority of the cases are found among people born before 1992, the year that hepatitis B vaccination began in newborns.”
“Most people with hepatitis B rarely show symptoms, so they don’t know they have it, and don’t get treatment early, so, they may be unwittingly spreading hepatitis B,” Prof. Dr. Pisit said.
Therefore, early diagnosis of this disease is crucial in stopping the spread of the infection and helping the patient to enter the treatment process timely, thus reducing the likelihood of cirrhosis and liver cancer, which are mainly caused by the hepatitis B virus in Thai people (read more in the Getting to Know Hepatitis B section).
The Pain point that started this research
Prof. Dr. Pisit explained that today, standardized tests for hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg), require the expertise of personnel and the use of large machine-based assays, which are often available in large hospitals and expensive, so access to the tests is still relatively limited and scarce in remote areas.
Inadequate access to screening for this disease is a key problem and a pain point that has sparked the development of this research and innovation.
“We are trying to develop a test kit that allows healthcare professionals at small hospitals or public health centers to screen for hepatitis B by themselves. The product we are developing is a self-test kit that can be self-administered or point-of-care testing that can be used at different care points to a certain extent. It makes screening patients more convenient while giving accurate results similar to machine-based testing,” Prof. Dr. Pisit explained.

Highlights of the Wireless Hepatitis B Test Kit
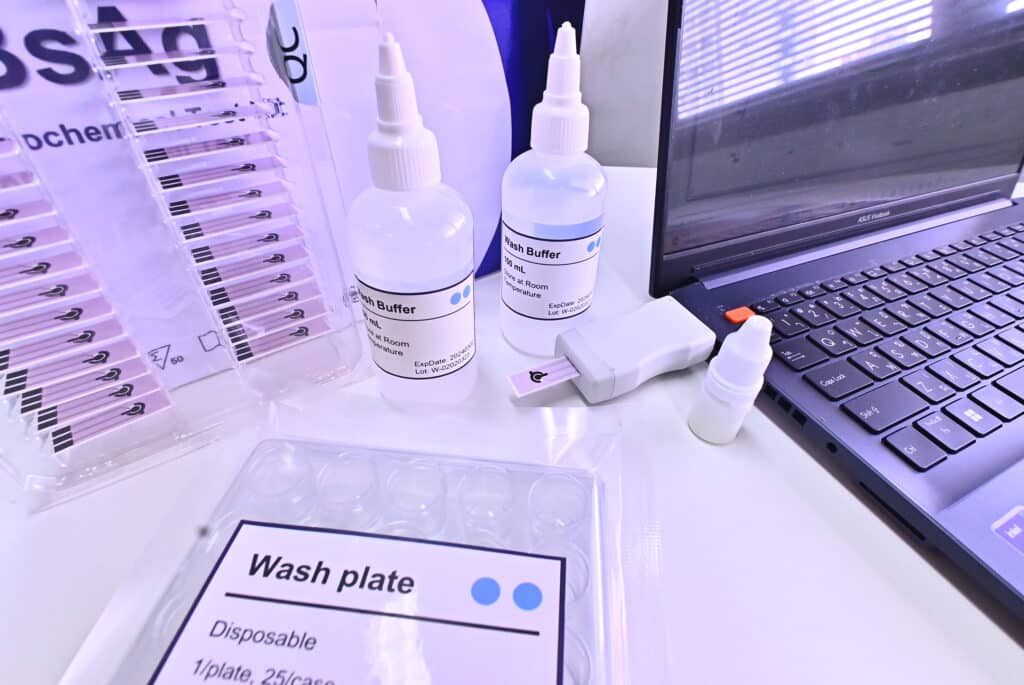
Prof. Dr. Pisit provides information on the wireless hepatitis B test kit that though there are now commonly used strip tests similar to the COVID-19 screening kits imported from abroad, the advantage of the product that the research team is developing is that in addition to getting quick results, the test can give an approximate quantity of virus, the data can be uploaded immediately, in real-time, and can be attached to the patients to whom the data belongs. For hepatitis B, it is very important to identify the patient because the treatment takes a long time.
The use of this test is different from the COVID-19 test, which is a strip test, also known as lateral flow, that is commonly available. Lecturer Dr. Natthaya, one of the research team members from the Faculty of Medicine, explained that the wireless hepatitis B test is an electrochemical biosensor based on the specific reaction between the antigen and the antibody. Once there is a binding between the antigen and the antibody, there is a change in electricity detectable by an electric current detector, also known as amperometric detection.
“The principle is that if there is a virus or antigen present, there will be a change in the current. The value of this measured current can be an indication of the amount of the antigen in a semi-quantitative way, and fluctuate according to the electricity current detected. “Simply speaking, other tests can only tell you if the antigen is “present or not present. But this test can not only tell if the infection is found or not but can indicate an approximate amount of found antigen as well. If the current is low, the antigen amount is high, and vice versa. It takes only short time to know the results and related information.”
For the screening procedure, only 2 µl (ml) of serum from the “blood sample” is used for dripping and incubation on the electrodes. Then, they are washed with wash buffer solution and wait for it to dry. It takes only up to 10 minutes for the results of the analysis through observation of the changes in electrical currents. “We need to use blood samples because other secretions will yield very small amounts of antigens and may not be as accurate as it could be,” Dr. Natthaya explained.
The Journey of Innovation to Address Public Health Problems
This innovative research and development are funded by the Program Management Unit for Competitiveness (PMUC) for 3 years. The project is divided into 3 phases from prototyping to commercialization.
Dr. Natthaya described the Phase 1 Research & Development which lasted throughout 2022.
“In the early years, we developed a prototype of a hepatitis B virus test kit using an electrochemical biosensors technique that works with a variety of measuring instruments. In the following year, for the kit to have reproducibility, and stability of current, with fast and convenient use, we developed the test kit with Bluetooth capability, so it can work both wirelessly and plugged into a small computer. The said Bluetooth is produced by a company in Taiwan. The current electrodes are produced in the lab of the Department of Biochemistry, Faculty of Medicine, Chulalongkorn University.”
Currently, the research has already entered phase 2.
“We’re now in the process of collecting data for field visits, including usage data and conducting clinical trials according to Section 27 of the Food and Drug Administration (FDA), exemption for research, and application for FDA certification before the production of commercialized kits ready for sale in the next phase,” Dr. Natthaya said, “We went through the first stage quite quickly because we had the know-how of electrochemical biosensors from Prof. Dr. Orawon Chailapakul and researcher Dr. Prinjaporn so it didn’t take long to further develop the prototype in the beginning.”
From research to practical innovation
In creating medical innovations, Dr. Natthaya recommends that researchers study and develop research in various stages following the requirements of various certifications, such as the FDA.
“In the past, we might have completed the research and waited until after its publication before applying for FDA certification. This may force some research papers to rest only on the shelf because they do not meet the FDA criteria. Currently, funding sources, especially external funding, are trying to push researchers to “do the reverse” by examining the FDA’s criteria and requirements before conducting research. Publication of the research is a bonus that will come later. This is so that such research can lead to practical innovation. ”
Prof. Dr. Pisit and Dr. Natthaya emphasized that for the development of medical devices to pass the FDA certification process, In addition to studying relevant requirements along with research development, there must also be an ISO 13485-certified production source for medical devices. Currently, there are two locations in Chulalongkorn University: the Faculty of Science and our laboratory at the Department of Biochemistry, Faculty of Medicine, which are used by the research team to produce the original electrochemical sensors as mentioned.
“Now it’s like we’re working for the FDA on the side as we try to constantly communicate with them in order to find out what they want. Medical innovation is very important and useful for our country. The easier access people have to healthcare, the faster they are cured, and the financial burden is drastically reduced.”
As for the future of wireless hepatitis B detection kits, Dr. Natthaya said that at the beginning of next year (2024), which is the beginning of phase 3, there should be a clearer direction for industrial mass production, which has already been roughly discussed with the private sector.
Prof. Pisit ended by saying, “This research is a collaboration of several researchers at Chulalongkorn University. I believe that there is still a lot of knowledge base that is good for society. This research is consistent with university policies that promote integration and cooperation by bringing knowledge and expertise from many sciences together to create great innovations that can meet society’s needs.”


Faculty of Medicine, Chulalongkorn University

